Research Projects
Time-Resolved Spectroscopy for the Detection of Biomolecules
One of the problems where we have focused our attention is in the detection of analytes in complex environments that are not suitable for standard fluorescence techniques. For example, many natural and non-natural media contain dyes that provide a fluorescent background that shadows the photoluminescence response of probes used to detect analytes in these samples. The approach that we have taken is the use of time-resolved photoluminescence spectroscopy.For example we have recently examined how to improve the detection limit of photoluminescent probes with long-lived excited states (Anal. Chem. 2012, 84, 8075). We have used these concepts (e.g. time-gating) to detect cysteine, homocysteine, and glutathione in complex autofluorescent media, using long-lived iridium complexes (Chem. Commun. 2012, 48, 11760). In addition, we have explored time-gating in the detection of protein aggregation (J. Am. Chem. Soc. 2011, 133, 11121) and RNA (Chem. Commun. 2009, 2640). In recent article (J. Phys. Chem. A 2014, 118, 10353) we reported on the use of time-resolved spectroscopy to differentiate between free-histidine and histidine bound to proteins by deconvoluting the complex time-resolved decay signals.
Some of these projects have been featured in news sources such as: BioOptics World, Photonics.com, E! Science News, and Science Daily.
Probing Protein Aggregation
We have found that ruthenium(II) dipyridophenazine complexes bind to amyloid-β fibrils with a concomitant increase in photoluminescence (J. Am Chem. Soc. 2011, 133, 11121). The transition of amyloid-β from monomers to fibrils is associated with the onset of Alzheimer’s disease. These metal complexes made available for the first time probes for the study of amyloid-β aggregation presenting large Stokes shift, red photoluminescence, and long lifetimes. Specifically, we used the long photoluminescence lifetime of these ruthenium complexes to monitor the amyloid-β fibrillization process even in the presence of a strongly autofluorescent background using time-gating. More recently, we used a combination of biophysical and computational techniques (in collaboration with Prabhakar group in the University of Miami) to study the binding of these complexes to amyloid-β fibrils (J. Am Chem. Soc. 2013, 135, 10810) and found that ruthenium dipyridophenazine complexes bind to a hydrophobic cleft in the surface of the amyloid-β fibril, between the aminoacids Valine18 and Phenylalanine20. Given the photoluminescence response of ruthenium dipyridophenazine complexes to protein aggregation, we studied α-synuclein aggregates in cells using the photoluminescence of ruthenium dipyridophenazine complexes (J. Am. Chem. Soc., 2012, 134, 20776) in collaboration with Segatori group at Rice University. These aggregates have been associated with the onset of Parkinson’s Disease. The unique properties of ruthenium dipyridophenazine complexes allowed the quantification of α-synuclein aggregation in H4 cells. We have also studied the aggregation of amyloid-β in real-time using fluorescent probes (ACS Chem. Neurosci. 2012, 3, 896), and amyloid-β in brain tissue using the birefringence of ruthenium red (ACS Chem. Neurosci. 2013, 4, 379) in collaboration with Murray group.
Research in this area has been featured in news sources such as: News on ruthenium complexes to track amyloid-β: Futurity, BioOptics World, Yahoo News, HighBeam Research, Science Daily; News on the binding of ruthenium complexes to amyloid-β: C&EN, Top News, Neuroscience News, Platinum Today, SciGuru; News on ruthenium complex for sensing α-synuclein aggregation: Medical Xpress, Platinum Today, PFHC Bulletin, LFW, Science Daily.
Dispersion of Single-Walled Carbon Nanotubes using Metal Complexes
The dispersion and disaggregation of as-grown SWCNTs in aqueous solution remain a significant obstacle to their use in most applications. One of the most common methods for dispersing SWCNTs in water involves the use of surfactants and polymers such as DNA or PVP. The dissolution of SWCNTs in organic solvents has also been studied being NMP one of the most efficient solvents. Non-covalent functionalization of carbon nanotubes (CNTs) is preferred to organic functionalization since the pristine state of SWCNTs is preserved. Therefore, we also study the dissolution of SWCNTs in aqueous solutions using metal complexes (Chem. Commun. 2011, 47, 2246). Ruthenium(II) complexes with ligands bearing extended aromatic systems are used to disperse and individualize SWCNTs by non-covalent interactions. The photophysical properties of these composites, specifically their photoinduced electron transfer reactions, make being studied using a variety of spectroscopic techniques that include photoluminescence spectroscopy, time-resolved photoluminescence spectroscopy and flash photolysis. Understanding how to synthesize materials containing SWCNTs, in which photoinduced electron transfer reactions can occur is of primary importance and will open new venues for CNTs applications.
News on photoactive ruthenium complexes to solubilize carbon nanotubes: Science Daily, Highbeam Business, Internet Chemistry, and EurekAler.
Nanomaterials: Synthesis and Photochemistry
The manipulation of nanometer-sized objects into ordered arrays is of fundamental importance to the design and synthesis of new materials. These materials generally possess new or amplified properties in comparison with disordered bulk systems. The organization of single-walled carbon nanotubes (SWCNTs) to form new materials is an intense area of research in carbon nanotechnology and pivotal for the development of technologies based on carbon nanotubes (CNTs). Important applications of such materials involve the fabrication of improved sensors, singlet oxygen generation, drug delivery, photodynamic therapy, photocatalysis, gas storage, and artificial photosynthesis, among others. A few years ago we reported a methodology to deposit individual SWCNTs on microporous and mesoporous silicate materials such as zeolites and MCM-41 using chlorosulfonic acid (Chem. Sci. 2011, 2, 1682). We actually showed that the protonation of SWCNTs on these silicate materials is reversible, with a recovery of their van Hove transitions, Raman spectra, and photoluminescence emission. Furthermore, we loaded ruthenium complexes inside the channels of the silicate materials and observed communication between these species and SWCNTs. Building on these discoveries we used chlorosulfonic acid to create thin transparent films of SWCNTs on quartz (ACS Nano 2012, 6, 5727). These films present tunable photoluminescent and electronic properties, depending on the packing density of the nanotube network. More recently, we used potassium metal to reduce carbon nanotubes and produce carbon nanotube polyelectrolyte dispersions that present liquid crystalline behavior (ACS Nano 2013, 7, 4503). These liquid crystalline solutions were used to manufacture macroscopic fibers with advanced optical, mechanical, and electronic properties (ACS Nano 2014, 8, 9107). On the other hand, we have also continued the research on photoactive metal complexes in materials. We encapsulated rhenium complexes within the supercavities of zeolite-Y (Angew. Chem. Int. Ed. 2013, 52, 12615). We discovered that this material presents vapoluminescence (change in photoluminescence intensity), luminescence vapochromism (change in spectral maximum), vapotemporism (change in lifetime) when exposed to solvent vapors, characteristics that were used to generate a “fingerprint” specific for each vapor.
News on carbon nanotubes-mesoporous silicates: Kurzweil, Vertical News, Machine like us;New on carbon nanotube polyelectrolytes liquid crystals: Azonano.com, Phys.org, Science Newsline, Science Daily; News on Carbon nanotube fibers: Science Newsline, R&D Magazine, EurekAlert. News on rhenium in zeolite-Y: Futurity, Science News, Science Daily.
Boron Nitride Chemical Functionalization and Dispersion
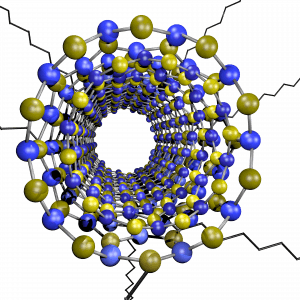 Boron nitride nanomaterials have just gotten the spotlight in the scientific community as they possess a unique set of properties that complement that of carbon nanostructures: they posses superb mechanical strength, high thermal conductivity, uniform wide band gap and high chemical and thermal resistance. However, their chemical inertness and low solvent-dispersibility have hindered the possibility to architect these nanostructures into functional macroscopic materials. Recently, our group has utilized the Billups-Birch reduction conditions to covalently functionalize boron nitride nanotubes (f-BNNTs) with aliphatic carbon chains. These aliphatic chains allow us to fully individualize BNNTs and help them disperse in solvents that otherwise wouldn’t be compatible with their precursor.
Boron nitride nanomaterials have just gotten the spotlight in the scientific community as they possess a unique set of properties that complement that of carbon nanostructures: they posses superb mechanical strength, high thermal conductivity, uniform wide band gap and high chemical and thermal resistance. However, their chemical inertness and low solvent-dispersibility have hindered the possibility to architect these nanostructures into functional macroscopic materials. Recently, our group has utilized the Billups-Birch reduction conditions to covalently functionalize boron nitride nanotubes (f-BNNTs) with aliphatic carbon chains. These aliphatic chains allow us to fully individualize BNNTs and help them disperse in solvents that otherwise wouldn’t be compatible with their precursor.
News on boron nitride nanotube functionalization: Science 360 News, Materials Today, Science Daily.